6.2 Chemical Bonds Vibrate
Elizabeth Johnson
Learning Objectives
- Describe the characteristics of vibrational movements of molecules and minerals.
- Determine the number of vibrational normal modes of a molecule.
Prior Knowledge and Skills
3.5 The Dual Nature of Electromagnetic Energy
3.7 Electromagnetic Energy: Units Conversion
Key Terms
- Vibrational normal mode
- Degenerate modes
Guided Inquiry
Watch the following animations of molecular vibrations:
How to use: click on the “i” button next to each vibration to bring up a video.
H2O: https://viva.pressbooks.pub/analyticalmethodsingeosciences/chapter/h2o-vibrations/
CO2: https://viva.pressbooks.pub/analyticalmethodsingeosciences/chapter/co2-vibrations/
SiO44- : https://viva.pressbooks.pub/analyticalmethodsingeosciences/chapter/sio44-vibrations/
These are examples of vibrational modes, or the vibrations that describe all possible internal motions of the molecule.
6.2.1. What is the difference in motion between a symmetric stretch and an asymmetric stretch?
6.2.2. Based upon the animations, do the different vibrational modes look like they have the same frequency (energy) or different frequencies?
6.2.3. Look at the symmetric bend for the CO2 molecule. In this figure, is the carbon atom moving in the y direction (vertically), or in the z direction (into and out of the page)?
6.2.4. If the CO2 molecule did a symmetric bend in the other direction, would you expect for it to have the same energy, or a different energy, from the symmetric bend in the animation?
The animations do not show degenerate modes, or multiple modes that have the same vibrational energy. We can calculate the total number of vibrational modes for a molecule:
Equation 6.2.1 Linear molecule: 3n-5
Equation 6.2.2 Non-linear molecule: 3n-6
6.2.5. How many vibrational modes does H2O have?
6.2.6. How many vibrational modes does CO2 have?
6.2.7. How many vibrational modes does a silicon tetrahedron (SiO44-) have?
Other Types of Vibrational Modes
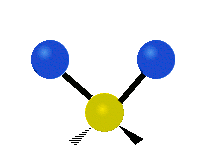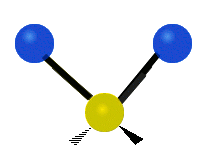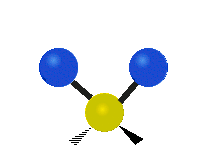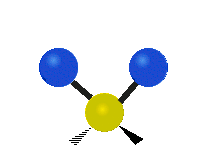
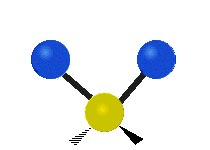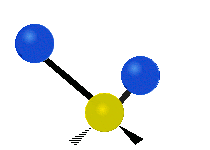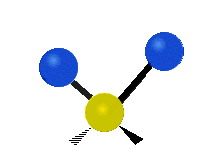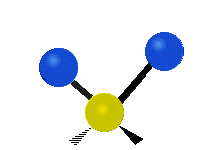
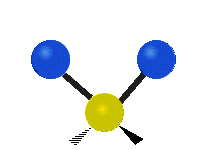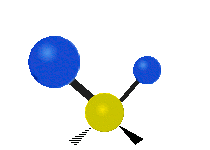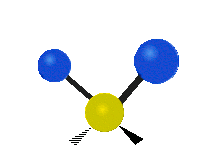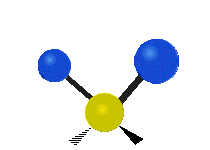
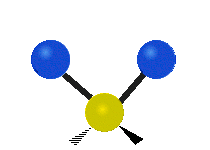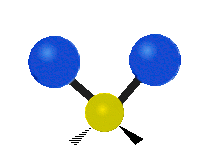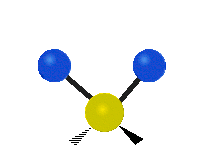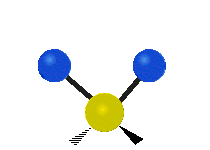
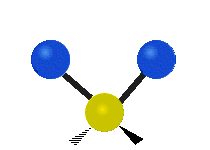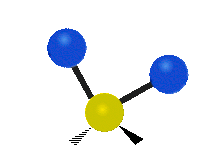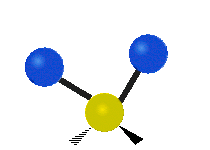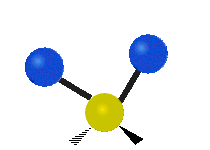
Vibrational modes are not limited to stretching and bending. Below, six types of molecular vibrational modes are shown.
 |
 |
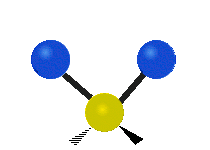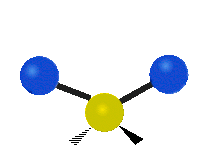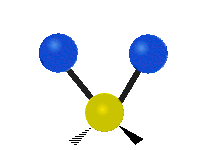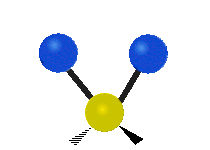 |
| Symmetric Stretch | Asymmetric Stretch | Scissoring or bending |
 |
 |
 |
| Twisting | Wagging | Rocking |
Figure 6.2.1 Six vibrational mode motions (Public Domain; Tiago Becerra Paolini).
6.2.8. What is the difference between “wagging” and “twisting” in terms of atomic motion?
Mineral Structures and Vibrations
What do vibrational modes look like in a solid such as a crystal structure?
Each link below is from the website “Animating the CRYSTAL vibrational frequencies” http://www.crystal.unito.it/animations-of-vibrational-modes.php.
Forsterite: http://www.crystal.unito.it/vibs/forsterite/
Calcite: http://www.crystal.unito.it/vibs/calcite/
Beryl: http://www.crystal.unito.it/vibs/beryl/
How to use: Choose the forsterite model. The middle column is a list of vibrational modes. Scroll down to 979.2 cm-1 (B1G is a symmetry designation) and click on that number. The mineral should start moving with that vibrational mode. In the left column, change the Si polyhedra to “transparent” so you can see the SiO44- motion.
Note that you can rotate the crystal structure by clicking and dragging your mouse on it.
6.2.9. Compare the 979.2 cm-1 mode to the 143.1 cm-1 mode. What is the difference in motion between these two modes? 143.1 cm-1 is an example of a “jello mode” – can you see why it might have this nickname?
6.2.10. In the calcite model, what is the difference in motion between 1400.1 cm-1 (EU) and 219.6 cm-1 (EU)? Hint: rotating the structure is helpful.
6.2.11. Choose two vibrational modes of beryl and describe how the motions are different from each other.
Summary
Molecular vibrations are quantized, and each molecule has a specific number of vibrational normal modes based upon the number of atoms and the geometry of the molecule. Minerals have more complex vibrational modes than molecules do, because of the vast number of atoms in a solid crystal.
References
Animating the CRYSTAL vibrational frequencies. (ret. 3.27.2019) http://www.crystal.unito.it/animations-of-vibrational-modes.php Libretext. Number of Vibrational Modes in a Molecule. (ret 3/27/2019) https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Spectroscopy/Vibrational_Spectroscopy/Vibrational_Modes/Number_of_Vibrational_Modes_in_a_Molecule Schauble, E. Characteristic Vibrations of CO2. (ret. 3/25/2019) http://www2.ess.ucla.edu/~schauble/MoleculeHTML/CO2_html/CO2_page.html Schauble, E. Characteristic Vibrations of H2O. (ret. 3/25/2019) http://www2.ess.ucla.edu/~schauble/MoleculeHTML/H2O_html/H2O_page.html Schauble, E. Characteristic Vibrations of CH4. (ret. 3/25/2019) http://www2.ess.ucla.edu/~schauble/MoleculeHTML/CH4_html/CH4_page.html Wikipedia. Espectroscopia de infravermelho (ret. 3/27/2019) https://pt.wikipedia.org/wiki/Espectroscopia_de_infravermelho

