2 Chapter 2 The Chemical Level of Organization
By Aylin Marz

Motivation. Every day nurses are subjected to chemicals including those found in disinfecting solutions, medications with toxic effects, various gases including those used in anesthesia, radiation producing chemicals used in treatment, as well as chemicals found in bodily fluids such as blood, saliva and urine. Generally, the doses or concentrations of these chemicals are too low to cause harm but with repeated exposures there is always additional risk of toxicity. Being aware of the benefit and harm of the chemicals nurses are exposed to can help reduce risk of developing diseases from these exposures.
The concentration of a chemical, as well as its chemical reactivity with the biomolecules within our bodies, plays a role in how it affects health. Therefore, understanding chemical reactions can help nurses avoid harm to self and patient. Different chemical characteristics allow for mixing, dissolving, or reactivity to occur between compatible chemicals. This concept is also important in nutrition and in understanding what type of biomolecules are needed for energy, healthy growth and development. Nurses informed about chemistry can help avoid harm and decide how to advocate healthy eating habits in their patients and communities.
Learning Objectives
Upon completion of the work in this chapter, students should be able to:
- Apply concepts related to atoms and elements to solve problems on isotopes
- Distinguish chemical reaction types such as hydrolysis degradation resulting in protein breakdown
- Perform an enzymatic reaction determining the reactants and products
- Apply the scientific method to determine the effect of factors affecting enzymatic activity
Background.
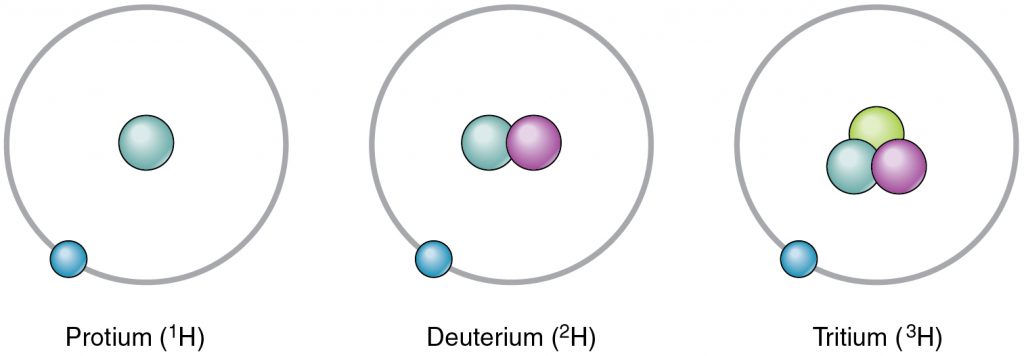
Life is made of chemicals which in turn are composed of various atoms composed of neutrons, protons, and electrons. There are many elements composed of atoms with different chemical properties each. Each element is determined by the number of its protons and this is called its atomic number. For example, Carbon is an element indicated with a C in the periodic table, and has an atomic number of 6. The number of protons and neutrons of an atom make up its atomic mass. There are multiple versions of an element based on its mass. For example, Carbon-12 has a mass number of 12 since it has 6 protons and 6 neutrons. Carbon-14 has a mass number of 14 since it has 8 neutrons. The different versions of an element are called its isotopes (Figure 2.2).
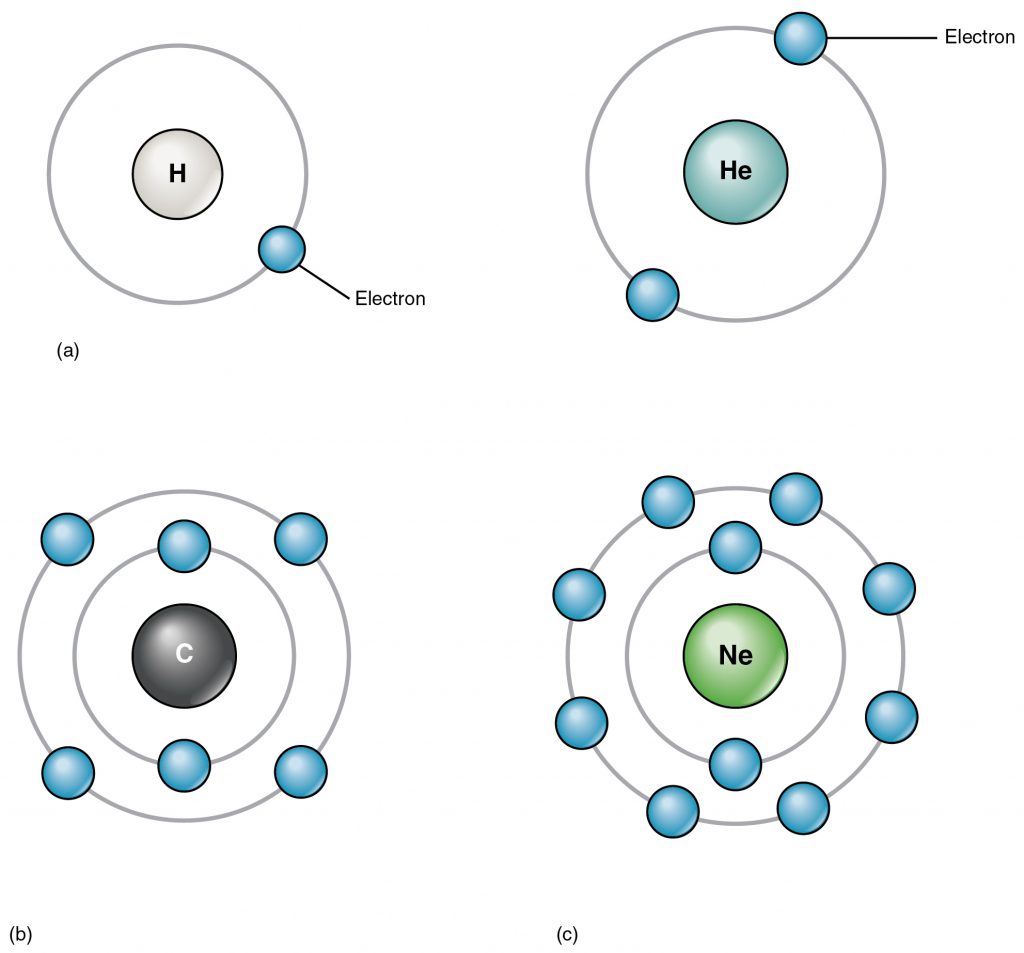
The electrons that orbit around the proton+neutron nucleus of an atom are negatively charged and are responsible for the interactions between different atoms. The negative charge of an electron is counteracted by the positive charge of the protons in what we call a neutral atom. For example, a neutral Carbon atom has 6 positively charged protons in its nucleus and 6 negatively charged electrons that orbit around the nucleus. The electrons of an atom are placed in orbitals (Figure 2.3). The first two electrons go in the innermost or first orbital, then the next 8 in the second orbital and the following 8 in the third orbital. The outermost orbital of an atom is called its valence orbital. The valence electrons are responsible for interactions between atoms that can result in a chemical reaction. The goal of a chemical reaction is to fill the valence orbital.
Chemical Bonds
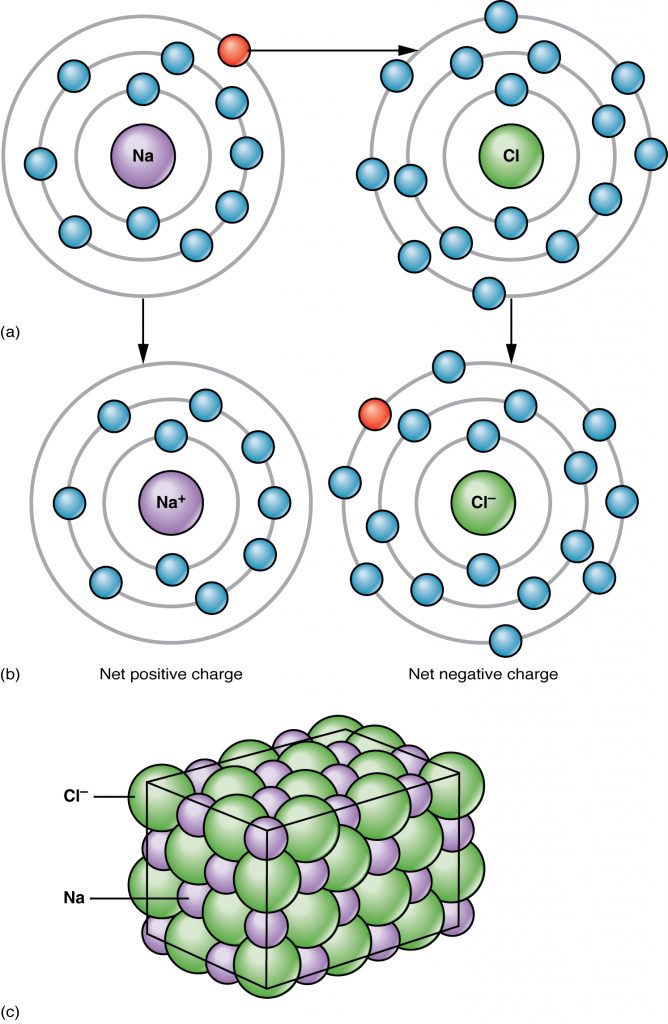
An atom can lose or gain electrons when it interacts with another atom. If an electron is lost, then the atom becomes positively charged ion due to the excess of protons and is called a cation. If electrons are gained, then the excess negative charge renders the atom an anion. Chemical reactions that result in transfer of electrons from one atom to another result in attractive forces between the atoms called ionic bonds In the example of the ionic bonding of Sodium (Na) and Chloride (Cl) occurs by transferring an electron from Na to Cl to produce NaCl which is table salt (Figure 2.4).
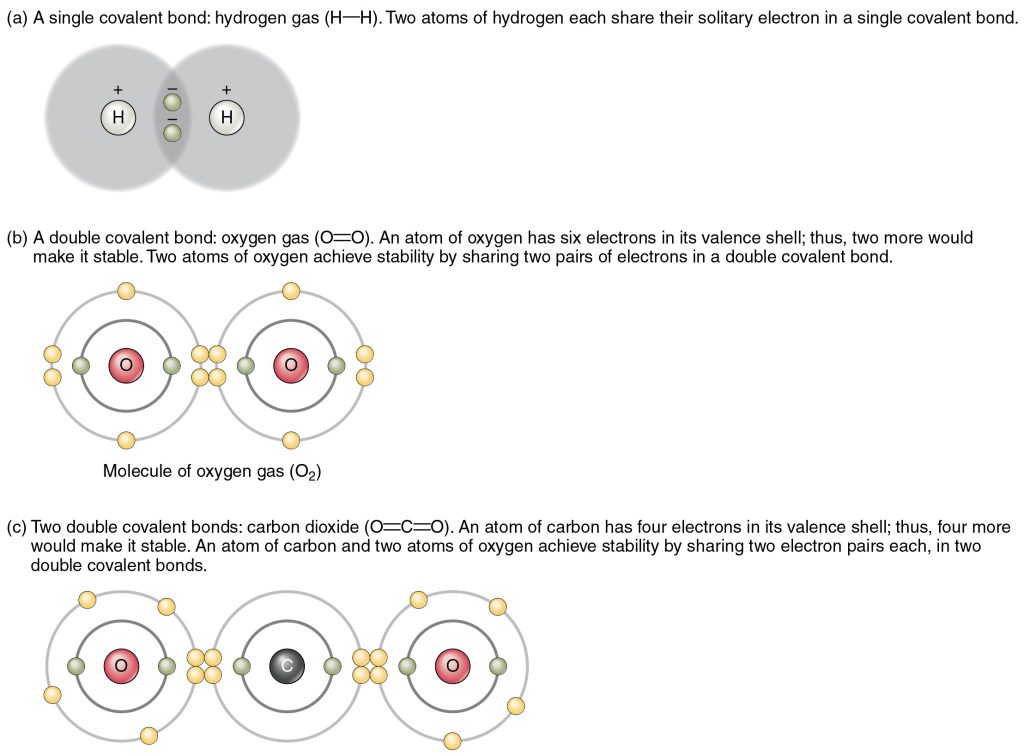
Chemical reactions that result in sharing of electrons between atoms form attractive forces that hold the two atoms together called covalent bonds. Formation of bonds between atoms produces molecules (Figure 2.5). For example, one carbon atom covalently bonded to four hydrogens produces a molecule called methane or CH4 that is a gas emitted by intestinal bacteria.
Many biologically relevant molecules are large and contain monomer units that are bonded together to form polymers. Proteins are made of amino acid monomers of which there are 20 different chemical varieties that
can be covalently bonded together (Figure 2.6). Carbohydrates contain monomers called monosaccharides such as glucose that are bonded together to form disaccharides such as maltose and polysaccharides such as glycogen. Nucleic acids, such as our genetic material DNA (deoxyribonucleic acid) is a long polymer made from monomers called nucleotides. There are many chemical types of lipids but the fats and oils also known as triglycerides are made from glycerol plus three fatty acids.
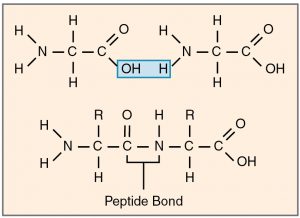
Proteins, carbohydrates, nucleic acids, triglycerides are molecules that are broken down during digestion into their monomeric subunits. These monomers are then absorbed into the blood and taken to cells that use them to produce energy (e.g. glucose) or to synthesize large molecules used in making cells structures. Making large molecules from their monomers require dehydration synthesis reactions in which one molecule of water is lost for each monomer bonded (Figure 2.6). When large molecules are broken down into their monomers, this requires hydrolysis degradation reactions in which a molecule of water is used to break each bond.
Any chemical reaction that occurs in a living organism requires the use of catalysts called enzymes. These are usually proteins that bind to the reactants or substrates and allow the reactants to interact more easily thereby reducing the amount of activation energy needed to start a reaction. To bind its substrate, an enzyme has to have a very specific three dimensional shape called its 3D structure. An enzyme has an active site that fits and binds its substrate like a lock and key. Upon binding, the shape of the enzyme changes slightly to make it bind its substrate even better. This is called the induced-fit model (Figure 2.6).
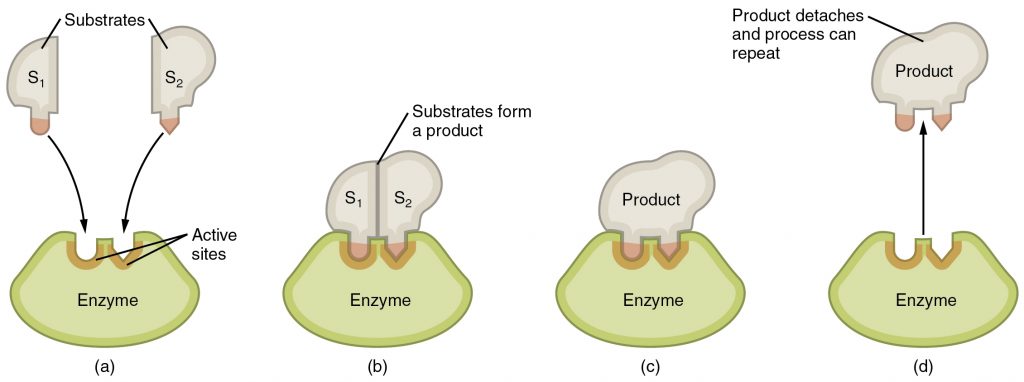
There are factors that affect the activity of an enzyme by modifying its 3D shape. These include co-enzymes such as vitamins, co-factors such as minerals, temperature, and the chemical environment in which the chemical reaction occurs such as the concentration of ions such as H+ which determine the pH. The pH of a solution is low if the H+ concentration is high and such a solution is called acidic. The pH of a solution is high if the H+ concentration is low and this is a basic or alkaline solution. Water has neutral pH. Enzyme shape or structure and activity is affected by pH. For example, stomach enzymes work at acidic conditions because the stomach is really acidic. These enzymes no longer work if they find their way into the intestine which is basic.
Pre-Laboratory Questions
After you review the background information, answer the following questions prior to attempting the laboratory exercises.
- What are the main subatomic particles making up an atom?
- Which subatomic particle is different among various isotopes of an element?
- What are the monomers that are covalently bonded to produce a protein polymer?
- How do enzymes function in chemical reactions?
- List some factors that affect the function of an enzyme.
Exercises
- Exercise 1. Radioactive Isotopes
- Exercise 2. Chemical Reaction – Breakdown of Collagen Protein
Exercise 1. Radioactive Isotopes
In the clinic, there are diagnostic methods that require giving the patient a radioactive isotope. This isotope can be traced and imaged in order to diagnose disease. What are isotopes and what makes them useful as tracers? Isotopes of an element have different atomic mass but the same chemical properties. Some isotopes are unstable and release high energy waves or particles that can be detected, followed and imaged, i.e. used as tracers of chemicals within the human body. These radioactive isotopes can also be used in treatment of disease usually because the high energy waves or particles they emit kill unwanted cells such as those of a tumor or a hyperactive thyroid gland.
In this exercise, we will explore the relationship between isotopes, atomic mass, and the number of subatomic particles using a simulation. The goal is to learn how mass number and atomic mass number (amu) relate to the number of protons, neutrons, electrons, as well as the abundance in nature of an elemental isotope. You will also see the relationship of all these to the stability of an isotope. Remember that radioactive isotopes that are useful in the clinic are the unstable isotopes.
Materials and Methods
The tool we will use is a simulation called “PhET Interactive Simulation: Isotopes and Atomic Mass”. Here is the link to access it: https://phet.colorado.edu/en/simulation/isotopes-and-atomic-mass.
- Once you get to this page, click on the arrow to begin playing the simulation.
- Then, select “Isotopes” (not Mixtures). You will get to a page that looks similar to the following Figure 2.7.
- You have the ability to view different elements by clicking on their symbol on the periodic table at the top right corner.
- You can add neutrons to the element by clicking and dragging one of the balls in the bowl named “Neutrons” onto the isotope. You can remove a neutron from the isotope by clicking on it and dragging it into the Neutrons bowl (bottom left corner).
- The isotope sits on a balance that allows you to select its mass number or atomic mass to view.
- The number of protons, neutrons, and electrons are displayed on the left top corner.
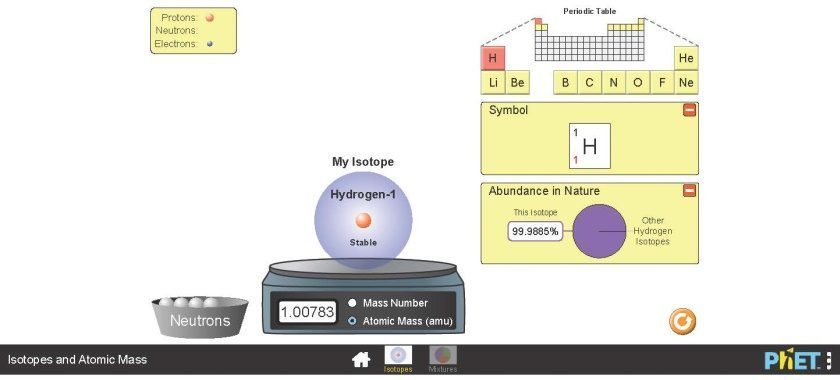
Exercise 1.1
Using the PhET interactive simulation fill out the information in the following table for the most abundant isotope of elements Carbon, Nitrogen, and Oxygen. The information for the element hydrogen is provided as an example.
| Element name | Element Symbol | Isotope Name | Isotope Symbol | % Abundance in Nature | Atomic Number | Mass Number
|
Atomic Mass (amu) | Number of protons, neutrons, electrons | Stability |
| Hydrogen | H | Hydrogen-1 | 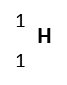 |
%99.9885 | 1 | 1 | 1.00783 | 1p, 0n, 1e | Stable |
| C | |||||||||
| N | |||||||||
| O |
Exercise 1.2
Select an element (other than hydrogen) from the periodic table shown in this simulation. Use the interactive simulation to answer the following questions regarding your element.
- What element did you select? List its name and symbol here. Example: Hydrogen, H.
- Which isotope of this element is the most abundant in nature? What is this % abundance? Example: Hydrogen-1, %99.9885
- By adding or subtracting neutrons from your isotope determine all of its isotopes that exist in nature. Use the following table to list the characteristics of each of these isotopes. Hydrogen is shown as an example.
| Isotope Name | Isotope Symbol | % Abundance in Nature | Atomic Number | Mass Number
|
Atomic Mass (amu) | Number of protons, neutrons, electrons | Stability |
| Hydrogen-1 | 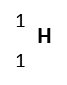 |
%99.9885 |
1
|
1 | 1.00783 | 1p, 0n, 1e | Stable |
| Hydrogen-2
(deuterium) |
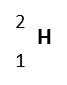 |
%0.0115 | 1 | 2 | 2.01410 | 1p, 1n, 1e | Stable |
| Hydrogen-3
(tritium) |
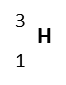 |
trace | 1 | 3 | 3.01605 | 1p, 2n, 1e | Unstable |
Exercise 1.3
For the following elements shown in the periodic table in this simulation determine their unstable isotopes. You can do this by adding or subtracting neutrons from your isotope (drag neutron balls from the bowl to your isotope to add, from the isotope to the bowl to subtract). For each unstable isotope, do a web search and determine if there is a clinical use for the isotope in diagnosis or treatment of patients. List the clinical use in the table below. Provide a web link to the information on clinical use.
| Element | Unstable / Radioactive Isotopes | Clinical uses | Web links |
| Hydrogen (H) | |||
| Boron (B) | |||
| Carbon (C) | |||
| Nitrogen (N) | |||
| Oxygen (O) | |||
| Fluorine (F) |
Exercise 1.4
At this point you have interacted extensively with the isotope simulation tool. Using your experience and observations come up with a hypothesis that relates the number of neutrons to the stability of the isotope. Design an experiment to test this hypothesis. Gather data using the simulation tool and record your data. Determine if your results support your hypothesis.
- Hypothesis: Based on your observation so far, what do you think is the relationship between the number of neutrons and the stability of an isotope of an element (any element, not just a specific one)?
- Experimental design: How would you use the simulation to test your hypothesis? What would you change (independent variable)? What would you measure/observe (dependent variable)? What do you compare your results to (control – Hint: Would this be the stable most abundant isotope for each element)?
- Results: When you changed your independent variable, how did that affect your dependent variable? You can use a table here to list each element you tested. You should test all the elements available to you.
- Conclusions: Did the results support your initial hypothesis about the relationship between the number of neutrons and isotope stability? If the results support your hypothesis, describe the supporting results. If the results did not support your hypothesis, indicate which results contradicted your hypothesis.
Exercise 2. Chemical Reaction – Breakdown of Collagen Protein
In this exercise you will use gelatin which is the component that makes Jello solidify when it cools down. Gelatin is made of a protein called Collagen which also exists as filler or extracellular matrix or connective tissue protein in our bodies. Both gelatin and collagen are made of chains of amino acids. For gelatin to do its function of solidifying juices when cooled, the amino acids have to remain covalently bonded to each other. If the peptide bonds holding the amino acids together break, then gelatin will not solidify the juices. You will use an enzyme called Bromelain in this exercise that catalyzes the breakdown of the covalent bonds between the amino acids of gelatin proteins.
Materials
- Gelatin (unflavored, e.g. Knox gelatin https://www.knoxgelatine.com/basics.htm)
- Incubator at 60oC
- Pineapple juice
- Transfer pipets
- Test tubes
- Test tube rack
- Test tube holder
- Test tube marker pen
- Heat block
- Beakers
- Glass stirrers
- Graduated cylinder
- Water
- Ice bath
Exercise 2.1 Preparation of the Substrate Gelatin Solution.
Follow the instructions on the gelatin package to prepare a gelatin solution that is two times more concentrated than instructed. We will call this 2X gelatin in water. For example if the package instructions say to add 100 mL of water, you add 50 mL instead (1/2). You will need boiling water, a beaker, and a graduated cylinder. Once the gelatin is dissolved, you can use it right away. Make sure to keep it warm in the 60oC incubator until you are ready to use it.
Question: After you add boiling water to gelatin, do you expect the three dimensional structure of gelatin protein to be maintained? If yes, why? If not, why not? Sketch a model of what you anticipate gelatin protein structure to be before and after adding the boiling water.
Exercise 2.2 Determining Gelatin Solidification Conditions and Timeline.
The goal of this exercise is to determine how long it takes to solidify gelatin.
- You will need three tubes with gelatin (+G) as your experimental replicates and three tubes without gelatin (-G) as control.
- Place the tubes on the tube rack. Label three tubes as control 1, 2, 3 and three tubes as gelatin +G 4, 5, 6.
- Transfer 4 mL of water into each of the control tubes labeled 1, 2, 3.
- Transfer 2 mL of water and 2 mL of 2X gelatin into the +G tubes 4, 5, 6. Mix by gently swirling the solution in the tubes.
- Place the tubes in the ice bath.
- Observe the tubes at 5 minute intervals until gelatin solidifies. Swirl the tubes to see if the liquid moves or not.
- Record your observations in the table below.
| Tube | Time (min) | Solid? No, Yes, Partial |
| 1 | 0 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 1 | 5 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 1 | 10 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 1 | 15 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
Question 1. Did all your replicates in the experimental group for gelatin solidify at the same exact time? If yes, explain why you expected this to be the case? If no, what would be a reasonable explanation for why not? Sketch your tubes to show how the controls and the experimental samples looked different at the end of your observation.
Question 2. What is the independent variable in this experiment? What is the dependent variable?
Exercise 2.3 Testing the Effect of Bromelain on Gelatin Solidification.
The goal of this experiment is to determine the effect of the Bromelain enzyme on the solidification of gelatin. To achieve this, you will compare a control group in which Bromelain is absent to an experimental group in which Bromelain is present. Both groups will have gelatin under conditions that normally allow solidification as you worked out in Exercise 2.1 and 2.2. To achieve this goal, follow the steps below:
- You will need six tubes for this experiment. Place the tubes on a rack.
- Tubes 1, 2, 3 are control tubes that do not contain Bromelain. Measure 2 mL of 2X gelatin and 2 mL of water into each tube 1, 2, 3.
- Tubes 4, 5, 6 are the experimental group tubes. Measure 2 mL of 2X gelatin and 2 mL of pineapple juice (Bromelain) into each tube 4, 5, 6.
- Let the tubes sit either on benchtop or in a warm incubator for 10 minutes to allow Bromelain to work on the gelatin.
- Place all tubes in the ice bucket.
- Observe the tubes and record how long it takes them to solidify similar to what you did in Exercise 2.2. Make sure to continue your observation until your control tube contents solidify.
| Tube | Time (min) | Solid? No, Yes, Partial |
| 1 | 0 | No |
| 2 | No | |
| 3 | No | |
| 4 | No | |
| 5 | No | |
| 6 | No | |
| 1 | 5 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 1 | 10 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 1 | 15 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
Question 1. Did all your gelatin containing tubes solidify at the same time? If yes, is that what you expected? If no, explain why you expected this result? Sketch the control and experimental tubes to show their differences at the end of the experiment.
Question 2. In this experiment, what is the independent variable? The dependent variable?
Question 3. Sketch a model that shows the enzyme-substrate interaction and the chemical reaction that occurs in the tubes that contain Bromelain. What are the reactant(s)? Product(s)?
Post-laboratory Questions
- In Exercise 1, you explored the relationship between the number of neutrons and isotope stability. Using what you learned, explore Iodine which has an isotope used in the clinic. Specifically, radioactive Iodine-131 is used to treat overactive thyroid glands by killing cells. Use the periodic table and your knowledge of atoms and elements to answer the following:
- What is the symbol for Iodine?
- How many protons does Iodine-131 have?
- How many electrons does neutral Iodine-131 have?
- If Iodine-131 loses an electron, what will be the charge on it? Will it be an anion or cation?
- How many neutrons does Iodine-131 have?
- The most abundant and stable isotope of Iodine is Iodine-127. Which subatomic particle number is different between Iodine-127 and Iodine-131? How many more or less of this particle does Iodine-131 have compared to Iodine-127?
- In Exercise 2, you showed that pineapple juice contains sufficient Bromelain enzyme to digest the collagen protein used preventing collagen from solidifying or gelling. Bromelain can work on other proteins as well. In fact, Bromelain purified from pineapple is sold in pill form as well as in cream form for various health benefits. Below you have some applications for which these pills and creams are used. For each scenario below, describe how you think Bromelain’s protein degradation activity could help achieve these therapeutic effects. Then, look up information online to help you better understand how Bromelain helps with these treatments. List your reference(s).
-
- Applying Bromelain cream on burns under a wound dressing:
- Taking Bromelain to aid in digestion:
- Bromelain taken to reduce cancer cell growth:
- Taking Bromelain tablets to reduce blood clot formation thereby reducing plaques in arteries in cardiovascular disease:
- Using Bromelain tablets to reduce inflammation in sinuses:
- Taking Bromelain to help with diarrhea:
- In Exercise 2, you used pineapple juice that contains the enzyme Bromelain. You allowed this enzyme to degrade or digest collagen which is a large protein. You then explored some physical factors such as temperature and chemical factors such as pH that affect enzyme activity. The source of your Bromelain was fresh pineapple juice. Given the factors that can affect enzyme activity, a group of nursing students get curious about the activity of Bromelain from fresh pineapple juice compared to canned pineapple juice. They set up an experiment to explore this question. Answer the following regarding the experimental design that will help compare Bromelain enzyme activity from fresh juice compared to canned juice:
-
- State the hypothesis that is being tested:
- What is the independent variable being altered in this experiment?
- What is the dependent variable being measured in the experiment?
- To set up the experiment you are given three groups of tubes. In each set, there are three tubes. All tubes contain the same collagen solution. In the space below, state what else you would add to each of these 9 tubes.
| Group 1 | Group 2 | Group 3 |
| Tube 1. | Tube 1. | Tube 1. |
| Tube 2. | Tube 2. | Tube 2. |
| Tube 3. | Tube 3 | Tube 3. |
-
- If the canned pineapple juice contains Bromelain that is less active than the enzyme from the fresh pineapple juice, what results would you expect?
- What are some reasons that may render the Bromelain from the canned pineapple juice to be less active than the Bromelain enzyme from the fresh pineapple juice?
The amount of medication taken at one time.
The amount of solute (chemical) dissolved in a solvent.
Ability of a chemical to hurt or harm the health of a person.
The ability of one chemical or element to react with another. It involves breaking and remaking of chemical bonds.
Organic molecules such as carbohydrates, proteins, lipids and nucleic acids that are important for energy, growth, and development of a person.
The change that occurs when one chemical evenly disperses within another. For example, when you dissolve salt in water the salt molecules (solute) become evenly dispersed in the water solvent.
This is the entity that is released from biomolecules used as food molecules such as carbohydrates. It is used in all bodily functions. It can be stored in chemical bonds and released through chemical reactions.
Increase in the number of cells within the body of a person. This occurs by cell division.
The changes that occur from when an egg is fertilized to the formation of a fully formed human being.
Atoms of the same element that have a different number of neutrons and therefore a different mass. Some isotopes are unstable and emit particles or waves rendering them radioactive.
Negatively charged subatomic particles with negligible mass.
An atom in which the number of negatively charged electrons equals the number of positively charged protons. A neutral atom has no net charge.
The outermost orbital of an atom carrying electrons.
An event that results in changing the attractive forces or bonds between atoms.
An atom that has net positive or negative charge.
An atom that has net positive charge.
An atom that has net negative charge.
Attractive forces formed between a positively charged ion and a negatively charged ion. These bonds are formed by electron transfer.
Chemical attraction due to sharing of electrons between atoms.
One atom chemically bonded to one or more other atoms.
A carbohydrate that contains two monosaccharides bonded together. E.g. two glucose monomers bonded together form the disaccharide maltose. Glucose plus fructose produce the table sugar sucrose which is a disaccharide as well.

